Our main task is to identify and understand the molecular modules that regulate the polarity and morphogenesis of plant cells. We focus on those that act at the interface between the secretory pathway, plasma membrane lipids and the actin cytoskeleton. We divide our attention between the two main processes that give plant cells their shape: orientated cell division and differentiated cell growth, and we focus on intracellular molecular mechanisms that control cell morphogenesis, such as exocytosis. (Detailed information can also be found on our website www.cell-biology.cz)

Our research
The organisation of cellular processes in eukaryotes depends on the transport of extracellular matrix, membrane lipids and proteins to the cell surface, which is mainly mediated by exocytotic vesicles. The secretory pathway culminates in exocytosis, a finely tuned process in which secretory vesicles are tethered to the plasma membrane, attached and fused, and finally release their cargo.
At the centre of our investigations is the exocyst, a conserved octameric protein complex that plays a central role in tethering secretory vesicles to the plasma membrane and thus acts as a key regulator of exocytosis. Our research focusses on investigating the functional aspects of exocyst function in a variety of plant species, from the angiosperms Arabidopsis thaliana and Nicotiana tabacum to the bryophytes Physcomitrium patens and Marchantia polymorpha and the streptophyte alga Klebsormidium nitens. An important aspect of our research is the investigation of the role of vesicular transport, in particular the exocyst, in the response of plants to abiotic stress and in the interaction between plants and microorganisms.
At the same time, our research focusses on the role of negatively charged phospholipids in the establishment and maintenance of plant cell polarity. Although these anionic lipids are only present in very low concentrations, they are of central importance for the control of membrane properties, their charge, curvature, signalling and protein recruitment. Of particular interest is the regulatory role of these phospholipids in the control of vesicular traffic at the plasma membrane. In this context, we also investigate lipid kinases and phospholipases that catalyse the production of the negatively charged phospholipid phosphatidic acid at the plasma membrane.
Significant results 2021-2023
Molecular insights into the function and evolution of the exocyst complex in plants
We have revealed the modular structure of the exocyst complex and precisely determined the role of the EXO70A1 subunit in anchoring the plant exocyst to the lateral plasma membrane in roots. This binding is mediated by interactions with a number of anionic phospholipids. Using a multi-faceted approach involving biochemical, genetic, microscopic and computational techniques, we have demonstrated the role of phosphatidylinositol-4-phosphate and phosphatidic acid in controlling exocyst attachment to the plant plasma membrane (Fig. 1). This study has also revealed the key role of membrane charge in controlling the interactions between peripheral proteins and membranes (Synek, Pleskot, Sekereš et al. 2021).
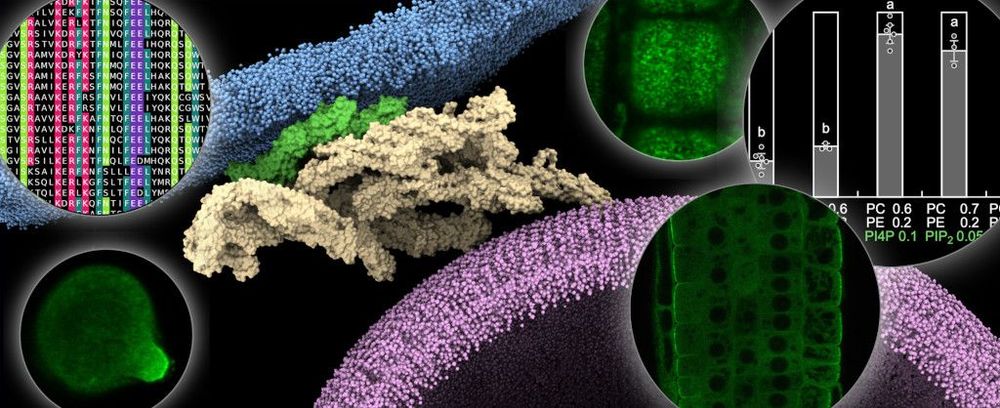
Figure 1: Model showing how the exocyst complex in Arabidopsis connects the secretory vesicle to the plasma membrane via the EXO70A1 isoform and several negatively charged phospholipids. The insets show the versatile methodological approach of our laboratory, combining state-of-the-art microscopic, computational, biochemical and in planta techniques. Inspired by the results published in (Synek, Pleskot, Sekereš et al. 2021)
We have elucidated the evolutionary history of the exocyst complex of land plants and uncovered a multistep evolutionary pattern of its different subunits. We have analysed the evolution of the EXO70 subunit and uncovered its division into three well-established subfamilies, each with unique functional properties. Using cross-species complementation analyses, we have revealed the extensive conservation of the canonical EXO70.1 subfamily and the independent evolution of the non-canonical EXO70.2 and EXO70.3 subfamilies (Haluška, Janková-Drdová et al., submitted). In addition, our study of the isoforms of the exocyst subunits SEC15a and SEC15b has shed light on their major roles in the male gametophyte and sporophyte and revealed a surprising acquisition of a new function for SEC15a in the sporophyte (Batystová et al. 2022). Our functional study of the exocyst in the moss Physcomitrium patens has provided insights into the importance of the exocyst subunit SEC6 for cell division and the organisation of multicellular structures. Our observations demonstrate the role of the exocyst complex in the transition from simple filamentous structures to complex morphological organisation in plant organs (Fig. 2) (Brejšková et al. 2021).

Figure 2: Disruption of the exocyst complex in the moss Physcomitroium patens leads to severe developmental defects. Taken from (Brejšková et al. 2021)
The role of vesicular transport in interactions between plants and microbes
In response to pathogenic fungi, plants use intense exocytosis to deposit cell wall stiffeners (papillae or so-called encasements) that prevent the spread of infection within the plant. We found that the exocyst complex containing the EXO70B2 subunit plays a crucial role in the membrane domains of papillae, which are important for callose deposition and the formation of cell wall incrustations. Our results suggest that the exocyst with the EXO70B2 subunit transports the important SNARE protein SYP121 into the membrane domains of the papillae, providing new insights into the mechanisms of resistance to penetration. (Ortmannová et al. 2022). We have found that the closely related exocyst subunit EXO70B1 plays a crucial role in the response to various abiotic stress factors and acts in a complex mode of cooperation/competence with the EXO70B2 subunit both in endomembranes and at the plasma membrane (Drs et al. in preparation).
Our research has elucidated the cellular processing, transport, localisation, secretion and function of the PR1 (pathogenesis-related 1) protein. By generating a spectrum of labelled PR1 variants in Arabidopsis, we have gained insights into the dynamic modulation of defence responses and immune pathways by PR1 protein processing. This complex interplay depends on cellular localisation and plant age and illustrates the versatility of this important plant defence protein (Pečenková et al. 2022).
To uncover the link between cell morphogenesis and biotic stress responses, we started to investigate how the immune stimulant chitosan influences the polar growth of plant cells. We found that chitosan induces callose deposition in root hairs and growth inhibition. Our results showed that this strategy is widespread in analogous root hair-like structures in lycopods and bryophytes, highlighting the importance of this response in situations of mild biotic stress. (Drs et al., submitted).
Lipid signalling at the plant plasma membrane
The growth of the pollen tube, a key component of plant sexual reproduction, requires a tightly regulated mechanism of pectin secretion to maintain the plasticity of the cell wall. Phosphoinositides and phosphatidic acid are involved in this regulation at the apical plasma membrane, but the processes controlling their production remain unclear. In collaboration with the group of Till Ischebeck at the University of Göttingen, Germany, we have shown that diacylglycerol kinase 5 plays an important role in the regulation of pectin secretion in apically growing pollen tubes (Fig. 3) (Scholz, Pejchar et al. 2022).
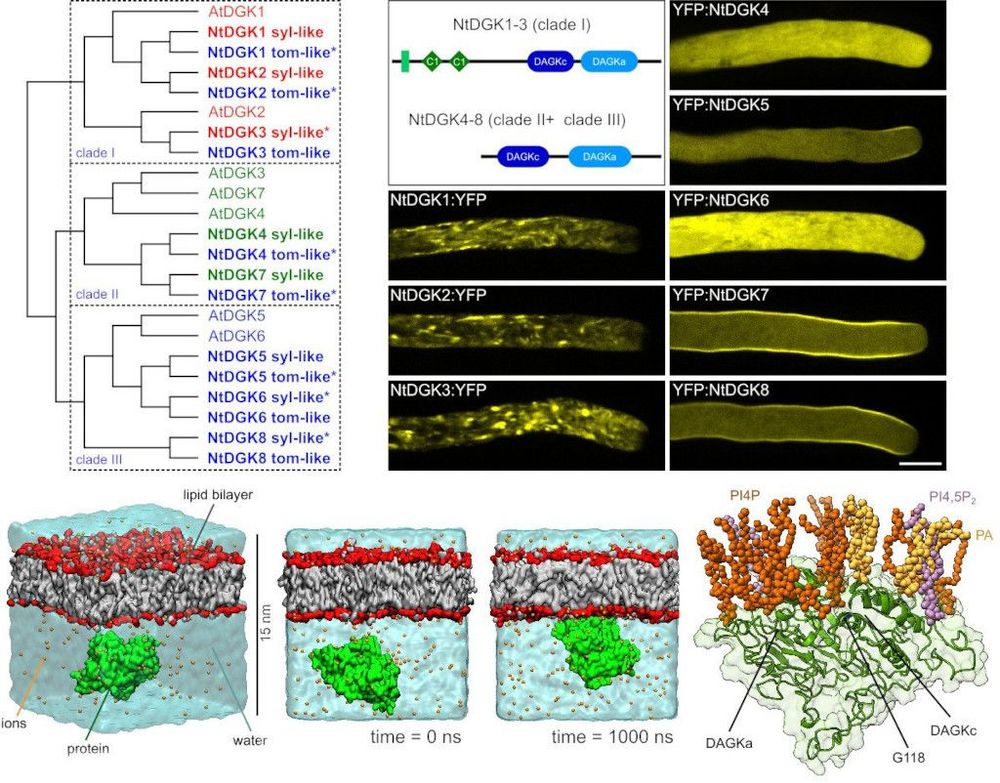
Figure 3: The isoforms of tobacco diacylglycerol kinase show different localisation patterns in the pollen tubes and bind to the plasma membrane via phosphoinositides. Left: Phylogeny of the diacylglycerol kinase (DGK) isoforms of Arabidopsis and Nicotiana tabacum and their categorisation into three clades. Right: Domain distribution and localisation of YFP-labelled NtDGK1-8 in actively growing pollen tubes. The lower panel shows molecular dynamics simulations showing mechanistic details of the interaction of NtDGK5 with the membrane. Scale bar 10 μm. Taken from (Scholz, Pejchar et al. 2022).
Our laboratory has been involved in two large collaborative studies led by Daniel Van Damme (PSB VIB Ghent, Belgium) and Roman Pleskot (Laboratory of Integrative Structural Biology, IEB) to decipher the role of anionic lipids in the regulation of clathrin-dependent endocytosis, in particular the structure and regulation of the TPLATE complex (TPC). We have mainly focused on the lipid binding properties of different TPC subunits and have shown that anionic lipids play distinct but complementary roles in the recruitment of TPC to the plasma membrane (Yperman et al., 2021a, 2021b). We have recently found that anionic lipids play a role in the biogenesis of biomolecular condensates of certain TPC subunits (Dragwidge et al., submitted).
Since membrane lipids play an important role in the response of plants to various stress factors, we have investigated to what extent lipids are involved in the perception of flagellin signalling, one of the cornerstones of plant immunity triggered by so-called molecular patterns (MAMPs). In collaboration with the Laboratory of Plant Pathophysiology and the group of Eric Ruelland (CNRS, Compiègne, France), we found that the flagellin-derived peptide flg22 triggers rapid and transient changes in the lipid dynamics of Arabidopsis plants. We identified diacylglycerol kinase 5 (DGK5), an enzyme that produces phosphatidic acid, as the gene responsible for these changes. Mutant dgk5.1 plants with impaired DGK5 function produced less phosphatidic acid in response to flg22 and showed reduced resistance. The enzymatic activity of DGK5, which is localised in the plasma membrane, is therefore essential for flagellin signalling and early immune responses in plant-microbe interactions (Kalachova, Škrabálková et al., 2022).

Figure 4: Examples of journal covers from 2021-2203 that refer to the results of our work (Batystová et al. 2022, Synek et al. 2021, Markovic et al. 2020)